There is a general perception in the wound healing products industry, that an intact tissue derived membrane can be used as a biocompatible collagen membrane. Unfortunately, this is a wrong concept. Please realize the fact, if the cells in any of the biologically intact membrane are washed off, it will not become non-immunogenic. The membrane will still retain elastin, type-III collagen, lipids and other proteoglycans which are all highly immunogenic. In order to reduce the immunogenicity of such adverse molecules, people end up with cross-linking. This results in potential blocking of surface chemistry, which is not good for exposing the clinically important features of the membrane like type-I collagen.
Currently, a tissue regenerative scaffold is not properly differentiated based on its chemistry. To clearly understand this Misconcept, let’s consider the applicable biomaterials used for tissue regenerative purposes (Ref 1)
- Synthetic polymers: Poly(α-esters), propylene fumarate, polymethylmethacrylate
- Synthetic ceramics:Calcium Phosphate Ceramics e.g. hydroxyapatite (HA), β-tricalcium phosphate (TCP)
- Biologically intact tissue-derived products: Made from membranes of amnion, pericardium, intestinal wall, urinary bladder etc.
- Bio-polymers:Collagen, fibrin, alginate, silk, HAc, and chitosan
Ideal characteristics of a biomaterial
Among all the above, the most ideal chemistry of the biomaterial used for tissue regeneration will have the following characteristics
- Cell and tissue acceptance (Biocompatibility): All the above-mentioned biomaterials are assumed to be biocompatible simply because of its chemical nature. But, on the other hand, a biomaterial made of denatured collagen (like gelatin) and the broken peptides of collagen or a biologically intact tissue-derived product should not be described as collagen products without realizing the biological impact of such molecules in terms of their bioactivity towards tissue regeneration and remodeling. A material is said to be biocompatible only if it is completely accepted as a natural, comfortable scaffold by the cells and the tissue involved in the granulation process without triggering any immunological response.
- Least rejection (Nil Immunogenicity): Certain aspects of immunogenicity are triggered by contaminants present in the biomaterial. Products derived from the membranes of amnion, pericardium, intestinal wall, urinary bladder etc. must be contaminated with approximately 15% elastin, type-III collagen, lipids and other proteoglycans which are all highly immunogenic. In order to reduce the immunogenicity, the biologically valuable type-I collagen contained in those products are chemically modified. This is not desirable as cross-linking will result in potential loss of bioactivity and other binding abilities that significantly impairs the tissue regenerative and wound healing abilities.
- Tissue/cell interaction (Bioaffinity): Tissue or cells interacting with the biomaterial should have an affinity towards it. In contaminated type-I collagen based biomaterials with unwanted cross-links, cells become non-responsive and unhappy due to the presence of contaminants like Type-III collagen, GAG’s (Glycos Amino Glycans) and Elastin. On the contrary, if a biomaterial is non-cross linked and free of contaminants, interaction with the underlying cells will trigger an improved cell signaling cascade to produce molecules for tissue repair and regeneration.
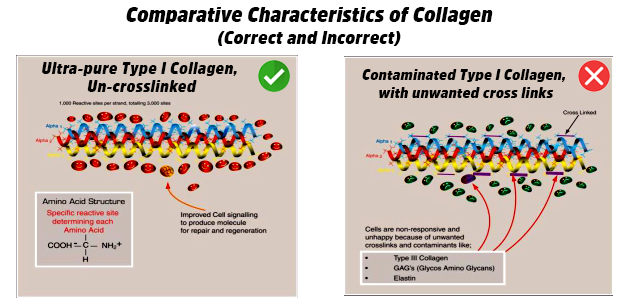
Conclusion: Use of a biomaterial with ideal characteristics like biocompatibility, non-immunogenicity and bioaffinity must be considered in the development of tissue regenerative scaffolds. It is not advised to choose a material that satisfies only the physical requirements of a medical scaffold. It is important that the biomaterial also meets the biomedical requirements much essential for the ultimate tissue regeneration and remodeling process.
Reference:
Lee, E. J., Kasper, F. K., & Mikos, A. G. (2014). Biomaterials for tissue engineering. Annals of biomedical engineering, 42(2), 323-337.
